Polyhydroxyalkanoates (Phas), Intracellular Pathways and Properties
Hasan Diba1 * , Reza Seifi-Kashani2 , Shohreh Tavakkoli2 and Saeid Malek-Mohammadi3
Corresponding author Email: hasan.diba@ymail.com
DOI: http://dx.doi.org/10.12944/CWE.10.Special-Issue1.78
Manufacturing of hard biodegradable petroleum based plastics harmfully affect the environment. Polyhydroxyalkanoates (PHAs) naturally produced as carbon storage polymer by various Monera kingdom microorganisms, resemble synthetic polymers in many chemical and physical properties. Renewable and biodegradable features made attracted much attention to these polyester bond polymers. PHAs extraction studied in many microorganisms made it naturally or engineered, where Poly 3-hydroxybutyric acid (PHB) is the most common. Main enzymes involved in PHB synthesis by Ralstonia eutropha encode by phbCAB gen cluster. Production of polyester particles is induced by excess quantity of carbon sources and nitrogen or some other factors starvation. PHAs production costs are still a drawback to wide usage, the future trend should focus on more efficient and economical processes developing for PHA production.
Copy the following to cite this article:
Diba H, Seifi-Kashani R, Tavakkoli S, Malek-Mohammadi S. Polyhydroxyalkanoates (Phas), Intracellular Pathways and Properties. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). DOI:http://dx.doi.org/10.12944/CWE.10.Special-Issue1.78
Copy the following to cite this URL:
Diba H, Seifi-Kashani R, Tavakkoli S, Malek-Mohammadi S. Polyhydroxyalkanoates (Phas), Intracellular Pathways and Properties. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). Available from: http://www.cwejournal.org/?p=10889
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-11-31 |
|---|---|
| Accepted: | 2014-11-04 |
Introduction
Manufacturing of plastics requires multiple amounts of petroleum; affect the environment harmfully (Bubacz and Goldsbery, 2014). The term “biomaterials” includes chemically unrelated products that are synthesized by organisms under different environmental conditions Luengo et al., 2003). Polyhydroxyalkanoates (PHAs) linear structure made biologically by diverse set of repeating ester bond units, where poly 3-hydroxybutyric acid (PHB) made by hydroxybutyrate units (Shakeri et al., 2011) is the most common (Singh and Parmar, 2011). PHAs accumulates water insoluble (Shakeri et al., 2011) carbon storage intracellular polymers (Sing and Parmar, 2011), have no osmotic pressure effects (Ksekdau et al., 2003).
PHAs naturally produced by various prokaryotic microorganisms including archaea and bacteria (Shakeri et al., 2011) either gram positive or gram negative (Elsayed et al., 2013) and some phototrophic cyanobacteria (McQualter et al., 2014; Schlebusch et al., 2013). They resembles synthetic polymers in many chemical and physical properties (Bagheriasl et al., 2012; Mullaney and Rehm, 2010), have been produced for use as bulk commodity plastics, fishing lines, and medical uses (Lu et al., 2009) such as pharmacy and drug delivery systems (Shakeri et al., 2011). PHAs have also attracted much attention as biodegradable polymers that can be produced from bio renewable resources (Lu et al., 2009).
PHA Synthesis Pathway
Our Knowledge about PHB production derived from Ralstonia eutropha (Jo et al., 2007) also known as Cupriavidus necator, Wautersia eutropha, and Alcaligenes eutrophus (Kocharin, 2012). A single chain translational fusion protein comprising three enzymes is required to establish the PHB biosynthesis pathway (Mullaney and Rehm, 2010). Enzymatic activities involved in PHB synthesis codes by phbCAB operon (Jo et al. 2007) as phbCAB gen cluster (Peralta-Gil et al. 2002). These enzymes are PHA synthase, NADPH-dependent acetoacetyl-CoA reductase, and β-ketothiolase encoded by phbC, phbA, and phbB genes, respectively (Ojume and Solomon 2003). Peralta-Gil et al. (2002) reported, in Azotobacter vinelandii the phbR transcriptional activator gene located upstream of phbBAC, belonging AraC family of activators (Peralta-Gil et al., 2002). The PHA synthesis can be summarized in eight pathways showed in fig. 1.
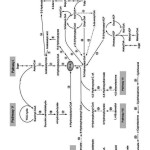 |
Figure 1: Biosynthesis of PHA pathways (Chen, 2010). |
The expression PHB synthesis is post transcriptional at the level of β-ketothiolase activity, which catalyzes the first step of PHB synthesis (Peralta-Gil et al., 2002). Glucose-6-phosphate dehydrogenase and isocitrate dehydrogenase are NADP+ regenerating enzymes as cosubstrate of acetoacetyl-CoA reductase, one of three key enzymes involved in the biosynthesis of PHB (Yamane, 1992). CoA inhibits β-ketothiolase activity under relaxed oxygen conditions by feeding acetyl coenzyme A into the tricarboxylic acid cycle. Under oxygen limitation and carbon excess conditions, NAD(P)H level increases, inhibits citrate synthase and isocitrate dehydrogenase. The level of acetyl-CoA and lowering the CoA has elevated by inhibiting these two enzymes, inhibition of β-ketothiolase by CoA is overcome and allows synthesis of PHB to proceed (Peralta-Gil et al. 2002).
Many genes encoding various enzymes directly or indirectly involved in PHA synthesis. Table 1 summarized pathways, abbreviation, and involved enzymes in PHAs synthesis.
Table 1: Synthesis pathways for PHAs (Chen, 2010).
|
No. |
Pathway |
Abbreviation |
Enzyme |
No. |
Pathway |
Abbreviation |
Enzyme |
|
1 |
Pathway I |
PhaA |
Β-ketothiolase |
16 |
Pathway V |
4hbD |
4-Hydroxybutyrate dehydrogenase |
|
2 |
PhaB |
NADPH dependent aceto-acetyl CoA reductase
|
17 |
OrfZ |
4-Hydroxybutyrate-CoA:CoA transferase |
||
|
3 |
PhaC |
PHA synthase |
18 |
Pathway VI |
Lactonase, putative |
||
|
4 |
Associated way |
PhaZ |
PHA depolymerase |
19 |
Hydroxyacyl-CoA synthase, putative |
||
|
5 |
PhaA |
Β-Ketothiolase |
20 |
Pathway VII |
Alcohol dehydrogenase, putative |
||
|
6 |
PhaB |
-dependent acetoacetyl-CoA reductase |
21 |
Pathway VIII |
ChnA |
Cyclohexanoll dehydrogenase |
|
|
7 |
PhaC |
PHA synthase |
22 |
ChnB |
Cyclohexanone monooxygenase |
||
|
8 |
Pathway II |
FabG |
3-Ketoacyl-CoA reductase |
23 |
ChnC |
Caprolactione hydrolase |
|
|
9 |
Epimerase |
24 |
ChnD |
6-Hydroxyhexanoate dehydrogenase |
|||
|
10 |
PhaJ |
(R)- Enoyl-CoA hydratas elenoyl-CoA hydratase I |
25 |
ChnE |
6-Oxohexanoate dehydrogenase |
||
|
11 |
Acetyl-CoA oxidase, Putative |
26 |
Semialdehyde dehydrogenase, Putative |
||||
|
12 |
Acetyl-CoA hydratase I, putative |
27 |
6-Hydroxyhexanoate dehydrogenase, putative |
||||
|
13 |
Pathway III |
PhaG FabD |
3-Hydroxyacyl-ACP-CoA transferase Malonyl CoA-ACP transacylase |
28 |
Hydroxyacyl-CoA synthase, putative |
||
|
14 |
Pathway IV |
NADPH-dependent acetoacetyl-CoA reductase |
|||||
|
15 |
SucD |
Succinic semialdehyde dehydrogenase |
Discussion
Bioplastics are an alternative substitute for petrochemical synthetic plastics (Ismail et al., 2010), preferred candidates for developing controlled release drug delivery vehicles and also can be used in biomedical implants and biofuels (Elsayed, 2013). Bioplastics are becoming increasingly prominent owing mainly to scarcity of oil, increase in the cost of petroleum based commodities, and growing environmental concerns with the dumping of non biodegradable plastics in landfills (Chen, 2014).
Cellular production of PHAs may be more “green” as compared to the use of specific metal catalysts for the production of polymers. The biosynthetic incorporation of specific monomers into PHA polymers is dependent on many factors that include the type of carbon source that the microorganisms are grown on (Lu et al., 2009). The culture conditions have main effects to induce PHA production, where different species of single genus don’t even have the same physiological response when exposed to the same culture conditions (Shakeri et al., 2011).
Production of polyester particles is induced when carbon source is in excess quantity or growth conditions have been imbalanced by declining other nutrient factors (Shakeri et al. 2011). PHAs extraction studied in many research from variety of microorganism including Azotobacter spp. (Khanafari et al., 2006), lactic acid bacteria (Ksekdau et al., 2003), Bacillus spp. (Chaijamrus and Udpuay, 2008), Ralstonia spp. (Shakeri er al., 2011), Azomonas sp. (Elsayed et al., 2013) Paracoccus denitrificans (Yamane, 1992), Azotobacter vinelandii (Peralta-Gil et al., 2002), Serratia sp. (Keshavarz and Roy, 2010), Sinorhizobium sp. (Shakeri et al., 2011), Enterobacter aerogenes (Aslam et al., 2013) and some engenieerd bacteria such as Escherichia coli (Mullaney and Rehm, 2010), Aeromonas hydrophila (Enan and Bashady 2004) and eukaryotic Saccharomyces cerevisiae (Kocharin, 2013).
The viability of microbial large scale production of PHB is dependent on the development of a low cost process. The commercial production of PHB has reported by using cheap substrates such as methanol, beet molasses, ethanol, starch, whey, molasses, and soy in articles (Wei et al., 2009). Khanafari et al. (2006) reported, PHB production by Azotobacter chroococcum on whey broth medium without extra salt can be was higher than other examined commercial media in their own research (Khanafari et al. 2006). Ksekdau et al. (2003) reported, Lactobacillus spp. produce the most PHB between lactic acid bacteria, where no significant correlation was observed between PHB production and the cell densities (Ksekdau et al., 2003). Chaijamrus and Udpuay (2008) had a research on PHB production by Bacillus megaterium utilizing commercial nutrients, concluded the highest production was observed after 45h of growth when 4% molasses and 4% corn sleep liquor respectively as carbon and nitrogen sources were used (Chaijamrus and Udpuay, 2008).
Conclusion
Establishing industrial biotechnology for the production of chemical compounds from the biosynthetic pathway has received a significant boost with the implementation of metabolic engineering, produce new products with higher yield and productivities (Kocharin et al., 2012). The increasing effects of non degradable plastic wastes is a growing concern environmental problems (Enan et al., 2004; Suriyamongkol et al., 2007). The versatility of PHAs has made them good candidates for the study of their potential in a variety of areas from biomedical fields to food, packaging, textile and household materials (Keshavarz and Roy, 2010). Renewable PHAs biodegradable macromolecule polyesters naturally produced by many species of prokaryotic microorganisms, these features make them superior to their petroleum based plastics rival.
Depending on PHAs microbial origin, bioplastics differ in their monomer composition, macromolecular structure and physical properties. Most of them are biodegradable and biocompatible, which makes them extremely interesting from the biotechnological point of view (Luengo et al., 2003). PHAs production costs are still a drawback to PHAs wide usage, the future trend should focus on more efficient and economical processes developing for PHA production, isolation, purification and improvement of PHA material properties.
References
- Aslam, R., Saleem, F. and Saleeem , Y. Biotechnological production of polyhydroxybutyrate (PHB) from Enterobacter aerogenes. Global Journal of Pure and Applied Sciences, 1(1), 1-8 (2003).
- Bagheriasl, S. Development and Characterization of Polyhydroxybutyrate from Selected Bacterial Species (University of Birmangam, 2012).
- Bubacz, M. and Goldsberry, A. Bioplastics Made from Industrial Food Wastes. Retrieved 1 January 2015 from: http://www.zeri.org/ZERI/The_Blue_Economy_files/20%20Biodegradable%20Plastics%20from%20food (2014).
- Chaijamrus, S. and Udpuay, N. Production and Characterization of Polyhydroxybutyrate from Molasses and Corn Steep Liquor produced by Bacillus megaterium ATCC 6748. Retrieved 24 December 2014 from: http://www.cigrjournal.org/index.php/Ejounral/article/view/1216 (2008).
- Chen, G. Q. Plastics completely synthesized by bacteria: polyhydroxyalkanoates, microbiology monographs. Microbiology Monographs, 14, (2010).
- Chen, Y. J. Bioplastics and their role in achieving global sustainability. Journal of Chemical and Pharmaceutical Research, 6(1), 226-231 (2014).
- Elsayed, N. S; Aboshanab, K. M; Aboulwafa, M. M. and Hassouna, N. A. Optimization of bioplastic (poly-β-hydroxybutyrate) production by a promising Azomonas macrocytogenes bacterial isolate P173. African Journal of Microbiology Research, 7(43), 5025-5035 (2013).
- Enan, M. R. and Bashady, S. A. PCR cloning of polyhydroxybutyrate synthase gene (phbC) from Aeromonas hydrophila. Arab Journal of Biotechnology, 7(2), 157-164 (2004).
- Ismail, I; Iskandar, N. F; Chee, G. M. and Abdullah, A. Genetic transformation and molecular analysis of polyhydroxybutyrate biosynthetic gene expression in oil palm (Elaeis guineensis Jacq. var Tenera) tissues. POJ, 3(1), 18-27 (2010).
- Jo, S. J; Maeda, M; Ooi, T. and Taguachi, S. Division of Biotechnology and Macromolecular Chemistry, Graduate School of Engineering. Journal of Bioscience and Bioengineering, 104(6), 457-463 (2007).
- Keshavarz, T. and Roy, I. Polyhydroxyalkanoates: bioplastics with a green agenda. Current Opinion in Microbiology, 13(3), 321–326 (2010).
- Khanafari A., Akhvan Sepahi, A. and Mogharab, M. Production and Recovery of Poly-β-Hydroxybutyrate of Whey Degradation by Azotobacter. Iranian Journal of Environmental Health Science and Engineering, 3(3), 193-198 (2006).
- Kocharin, K. Metabolic Engineering of Saccharomyces cerevisiae for Polyhydroxybutyrate Production (Göteborg, 2013).
- Kocharin, K; Chen, Y; Siewers, V. and Nielsen, J. Engineering of acetyl-CoA metabolism for the improved production of polyhydroxybutyrate in Saccharomyces cerevisiae. AMB Express, 2(52) (2012).
- Ksekdau, Z. N. Y; Beyatli, Y. and Aslim, B. Determination of Poly-hydroxybutyrate (PHB) Production by Some Mesophilic and Thermophilic Lactic Acid Bacteria. Turkish Journal of Biology, 27, 37-42 (2003).
- Lu, J., Tappel, R. C. and Nomura, C. T. Biosynthesis of Poly(hydroxyalkanoates). Polymer Reviews, 1, (2009).
- Luengo, J. M; Garcia, B; Sandoval, A; Naharro G. and Olivera, E. Bioplastics from microorganisms. Current Opinion in Microbiology, 6(3), 2541-2260 (2003).
- McQualter, R. B; Somleva, M. N; Gebbie, L. K; Li, X; Petrasovits L. A. and et al. Factors affecting polyhydroxybutyrate accumulation in mesophyll cells of sugarcane and switchgrass. BMC Biotechnology, 18(83), (2014).
- Mullaney, J. A. and Rehm, B. H. A. Design of a single-chain multi-enzyme fusion protein establishing the polyhydroxybutyrate biosynthesis pathway. Journal of Biotechnology, 147, 31-36 (2010).
- Ojume, T. V; Yu, J; and Solomon, B. O. Production of polyhydroxyalkanoates a bacterial biodegradable polymer. African Journal of Biotechnology, 3(1), 18-24 (2004).
- Peralta-Gil, M; Segura, D; Guzmán, J; Servín-González, L. and Espín, G. Expression of the Azotobacter vinelandii Poly-β-Hydroxybutyrate Biosynthetic phbBAC Operon Is Driven by Two Overlapping Promoters and Is Dependent on the Transcriptional Activator PhbR. Journal of Bacteriology, 184(20), 5672-5677 (2002).
- Schlebusch, M; Huge, J; Kopka, J. and Hagemann, M. Metabolic Changes in Synechocystis PCC6803 upon Nitrogen-Starvation: Excess NADPH Sustains Polyhydroxybutyrate Accumulation Waldemar Hauf. Metabolites, 3, 101-118 (2013).
- Shakeri, S; Roghanian, R. and Emtiazi, G. Comparison of intracellular polyhydroxybutyrate granules formation between different bacterial cell subpopulations by flow cytometry. Jundishapur Journal of Microbiology, 4(4), 229-238 (2011).
- Singh, P. and Parmar, N. Isolation and characterization of two novel polyhydroxybutyrate (PHB) producing bacteria. African Journal of Biotechnology, 10(24), 4907-4919 (2011).
- Suriyamongkol, P; Weselake, R; Narine, S; Moloney, M. and Shah, S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants - a review. Biotechnology Advances, 25(2), 148-175 (2007).
- Wei, X. X; Shi, Z. Y; Youn, M. Q. and Chen, G. Q. Effect of anaerobic promoters on the microaerobic production of polyhydroxybutyrate (PHB) in recombinant Escherichia coli. Applied Microbiology and Biotechnology, 82, 703–712 (2009).
- Yamane, T. Cultivation engineering of microbial bioplastics production. FEMS Microbiology Letters, 103(2-4), 257–264 (1992).






